Introduction
Carotenoids are one of the largest and most diverse groups of biologically active substances, which are responsible for yellow, orange, and red in many plants, microorganisms, and animals. These natural products can protect cells against harmful oxygen radicals or serve as a precursor of vitamin A, a membrane stabilizer (Asker et al., 2012). Carotenoids are an effective antioxidant due to the main structure of a conjugated double-bond carbon chain. Therefore, these natural pigments have received considerable attention for product development applied in biotechnology, the food industry, animal feed, aquaculture, and the pharmaceutical industry (Fiedor & Burda, 2014; Vílchez et al., 2011).
In bacteria, carotenoids are synthesized to protect bacteria against disadvantageous environments such as high temperatures, high UV intensity, or undernutrition conditions (Terao, 1989; Jorgensen and Skibsted, 1993; Stahl and Sies, 2003). In recent years, many Bacillus species have shown the ability to produce a variety of carotenoids, such as Bacillus atrophaeus producing black pigment; Bacillus cibi, Bacillus vedderi, Bacillus okuhidensis, Bacillus clarkii, Bacillus pseudofirmus producing yellow to orange pigment (Duc et al., 2006).
Materials and Methods
Bacterial samples: Bacillus samples were collected from soil and seawater at the seashore in Kien Giang Province of Vietnam. The isolates were identified by biochemical testing and 16S rRNA analysis.
Chemicals: Difco sporulation medium (DSM) and Luria-Bertani medium (LB) were purchased from Himedia, India. β-carotene, FeCl3, potassium ferricyanide (K3[Fe(CN)6]), 2,2-diphenyl-1-picrylhydrazyl (DPPH), CCl3COOH (TCA), and Silicagel (0.063 – 0.200 mm) from Sigma-Aldrich. Chloroform, Methanol 99.5%, phosphate-buffered saline (pH 7.4), and chloroform 99.5% were purchased from Merck, Germany. F-primer f27, R-primer r1492, DNA master mix, and PCR water were purchased from Phusa Genomics LTD. Company.Carotenoid extraction
Carotenoids were extracted based on the method described previously by Indra Arulselvi et al. (2014). One colony of each strain was pre-cultured in 10 mL LB medium, incubated at 37°C, and shaken at 200 rpm for 24 hours. Next, 2 mL of pre-inoculum was transferred into a 250 mL flask containing 100 mL of LB, incubated at 37°C, and shaken at 200 rpm for 48 hours. Cells were harvested by centrifugation at 5,000 rpm for 30 minutes at 4°C, and then the pellet was washed with distilled water. For carotenoid extraction, 6 mL of methanol: chloroform (1:2, v/v) was mixed with 0.2 gram of pellet, and the suspension was sonicated at 200 W at 4°C for 10 minutes, which was followed by adding 6 mL of distilled water into the tube. The suspension was separated by centrifugation at 12,000 rpm for 15 minutes at 4°C. The lower phase having carotenoids was collected. Extracted liquid was monitored at 400 to 600 nm by UV/VIS spectrophotometer (GENESYSTM 10S UV-Vis Spectrophotometer - Thermo Fisher). Total carotenoid content was calculated by the following formula (Liaaen- Jensen and Jensen, 1971):
![]()
Where c is total carotenoid content (mg); D is the maximum absorbance; v is the volume (mL); f is the dilution coefficient, 10 is the conversion factor to mg, and 2,500 is the specific extinction coefficient of carotenoid.
Antioxidant activity tested
DPPH assay
Antioxidant activity was measured by DPPH assay based on the method of Brand-Williams (1995) with modification. Carotenoid extracts (500 µL) were added to the tube containing 500 µL of DPPH solution (0.025 mg/mL in chloroform). All the solution in tubes was measured by the absorbance spectrophotometer at 517 nm after 30 minutes of incubation in dark conditions (against a blank, reaction mixture without DPPH).
Carotenoid extracts were prepared in different concentrations. Chloroform was used as the negative control. β-carotene (conc. 1-6 µg/mL) was used as a standard. All treatments were conducted in triplication.
The percentage of antiradical activity (%AA) was calculated:
![]()
Where: AbS: sample absorbance, AbC: control sample absorbance.
The antioxidant activity of carotenoid extracts was evaluated through the IC50 value which means the exact concentration that the extracts could inhibit or reduce 50% of DPPH radical.
Reducing power assay
The ability of extracts to reduce ferric ions (Fe3+) was assessed by the method of Oyaizu (1986) with modification. Particularly, 500 µL of carotenoid extract (1 mg/mL) was mixed with 500 µL phosphate buffer (0.2 M, pH=6.6) and 500 µL of a 1% potassium ferricyanide, then the mixture was incubated at 50°C for 20 min. About 500 µL of TCA 10% was added to the mixture. Finally, 1,000 µL of the solution was mixed with 1,000 µL of distilled water and 200 µL FeCl3 (0.1%). The absorbance was recorded at 700 nm against blank. All treatments were conducted in triplication.
β-carotene was used with different concentrations (0.2 – 1.0 µg/mL) as a standard curve. Methanol was used as a blank. The results were expressed as equivalent β-carotene concentration (µg/mL).
β-carotene identification
Purification of the carotenoid extract by silica gel chromatography column
The carotenoid extract was concentrated by using a rotary evaporator, and then a freeze dryer. The residue was dissolved in chloroform to get the final concentration at 10 mg/mL. This solution was subjected to a glass chromatography column on silica gel, equilibrated with chloroform. The column was eluted with different ratios of chloroform-methanol, (9:1, 8:2, 7:3, 6:4, 4:6, 3:7, 2:8, and 1:9 (v/v), respectively). Fractions were collected based on color and maximum absorbance measured by spectrophotometer from 400 – 600 nm.
Identification of β-carotene by HPLC – MS
Fractions collected from the silica gel chromatography column were identified by Agilent 1290 HPLC (USA) and micrOTOF-QII Bruker Daltonic mass spectrometry (Germany).
The column used was ACE3- C18 (4.6 x 150 mm, 3.5 µm), kept at 40°C and the flow rate is 0.1 mL/min. The injected volume was 20 μL. The mobile phase consists of component A (deionized water - containing 1% formic acid) and component B (methanol - containing 1% formic acid). The gradient changed from 10% to 95% solvent B, as follows: 0-2 min 10% solvent B, 2-20 min 10% solvent B, 20-30 min 95% solvent B, and the column was re-equilibrated with 0% solvent B for 5 minutes (Table 1).
Table 1. Parameters of mobile phase in HPLC
|
Time (min) |
% Phase A |
% Phase B |
|
0 |
90 |
10 |
|
2 |
90 |
10 |
|
20 |
5 |
95 |
|
30 |
5 |
95 |
Further, the fractions were analyzed with the micrOTOF-QII Bruker Daltonic mass spectrometer with Electrospray Ionization (ESI), a dual-ion channel funnel followed by a hexapole, an analytical quadrupole, in‐source collision‐induced dissociation (CID), high-resolution mass separator TOF, multichannel probe. Data were analyzed on Data Analysis software (Bruker, Germany).
The m/z ion accuracy was calibrated on the ESI – L tuning mix using a syringe direct sample pump at 200 μL/hr. The MS parameters are set up as follows: ESI parameters: capillary voltage 4500V, nebulizer pressure 1.2 Bar, dry gas 8 L/min, temperature 200oC; ion filter (Funnel) including voltage RF1 350V and voltage RF2 360V, ion isolator (Q); ionic energy 8eV, lowest mass spectrometry 100 m/z; CID with potential 8eV, RF: 300V, mass separator (TOF) with transfer time 120 m/s.
Statistical analysis
The data were processed by Microsoft Excel 2016 and analyzed using IBM SPSS Statistics 20 software for determining the differences among treatments.
Results and Discussion
Identification of bacterial isolate
There are many Bacillus samples isolated at the seashore in Kien Giang province of Vietnam. Isolate ND4-4 was collected from the soil of Nam Du islet in Kien Giang Province and was identified by biochemical test and 16S rRNA analysis. Colonies of ND4-4 showed pink color on Nutrient Agar (NA) (Figure 1), circular shape, undulated edge, and flat surface. Isolate ND4-4 was determined as Gram-positive, motile, able to form endospores, and catalase positive. Isolate ND4-4 was identified as Bacillus sp. according to the key Bergey’s manual of determinative bacteriology (Amin et al., 2012; Holt et al., 1977).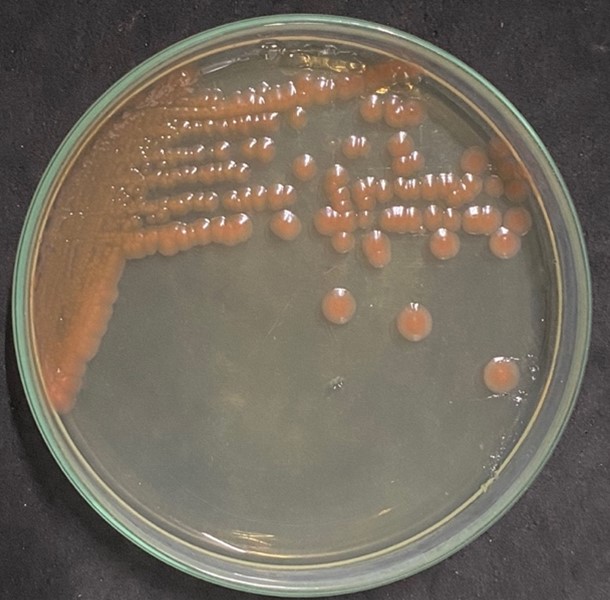
Figure1. Colonies of ND 4-4 on Nutrient Agar (NA)
The 16S rRNA sequence of ND4-4 was shown in Figure 2. The obtained 16S rRNA sequence of ND 4-4 was aligned to bacterial 16S RNA sequences recorded in the gene database (NCBI) by using BLAST software. The result showed that the 16S rRNA sequence of ND4-4 is 100% identified with Bacillus infantis strain BAB-2130 (JX486842.1).

Figure2. The 16S rRNA sequence of ND4-4
From morphology, biochemical properties, and 16S rRNA identification, it can be concluded that isolate ND4-4 was Bacillus infantis. Bacillus infantis was first isolated from blood samples or newborns (Ko et al., 2006). Currently, there are not many reports on carotenoid – producing ability of Bacillus infantis. Thao et al. (2011) investigated carotenoid - producing ability of several Bacillus strains, including Bacillus infantis, as a probiotic material providing carotenoids.
Carotenoid extraction
Carotenoid extract of Bacillus infantis ND4-4 in methanol: chloroform (1:2, v/v) showed red-orange color (Figure 3), and maximum absorbance at 460 nm. The total carotenoid content of carotenoid extract from Bacillus infantis ND4-4 after 24 hours cultured in LB, pH 7 is 1.044 mg.

Figure3. Carotenoid extraction of Bacillus infantis ND4-4
Antioxidant activity tested
DPPH Assay
The antioxidant activity of carotenoid extract by DPPH assay was evaluated by the percentage of antiradical activity (%AA) and the half maximal inhibitory concentration (IC50) value, the concentration of the sample at which the DPPH was 50% inhibited. A lower IC50 value indicated that the sample had a stronger activity and vice versa. This value was formulated through a linear equation of the % AA at given concentrations.
The DPPH radical scavenging ability of β-carotene increased with the increase in concentration (as shown in Table 2). Accordingly, β-carotene investigated at a concentration of 1 μg/mL could reduce DPPH radical by 26.6%, increasing linearly and reaching 96.5% at a concentration of 5 μg/mL. However, the percentage of antiradical activity at 6 μg/mL was not significantly different from 5 μg/mL (reaching 97.2%). The reason might be interpreted that at the concentration of 5 μg/mL, the amount of DPPH radical in the reagent has been mostly reduced, reaching >90%, and increasing in β-carotene concentration was not significant.
|
Table 2. DPPH radical scavenging activity of β-carotene |
|
|
Concentration (μg/mL) |
Antiradical activity (%) |
|
1 |
26.6±1.1e |
|
2 |
49.6±1.4d |
|
3 |
66.7±0.1c |
|
4 |
81.7±1.3b |
|
5 |
96.5±0.6a |
|
6 |
97.2±0.6a |
|
Figures are shown in the table as mean ± SD of triplicate. Different letters indicate a significant difference at p<0.05 by the Duncan post hoc test |
|
The data in Table 3 shows that crude extract from Bacillus infantis ND4-4 possessed DPPH quenching activity and followed a dose response.
Table 3. DPPH radical scavenging activity of carotenoid extract from Bacillus infantis ND4-4
|
Concentration (μg/mL) |
Antiradical activity (%) |
|
0.4 |
20.5±0.4g |
|
0.8 |
27.8±0.5f |
|
1.2 |
37.3±0.5e |
|
1.6 |
41.7±0.3d |
|
2.0 |
47.8±1.3c |
|
2.4 |
53.8±0.3b |
|
2.8 |
60.2±1.3a |
Figures are shown in the table as mean±SD of triplicate. Different letters indicate a significant difference at p<0.05 by the Duncan post hoc test.
The IC50 (half maximal inhibitory concentration) value used to compare DPPH radical scavenging activity among crude extract and β-carotene was calculated from the linear correlation of the antiradical activity (%) and carotenoid concentration (expressed as the total carotenoid concentration in the crude extract). The result of IC50 of β-carotene reached a 50% quenching of DPPH radical at 2.17 μg/mL. Bacillus infantis ND4-4 extract had a total carotenoid content/crude extract of 0.09% and the IC50 value at 1.92 μg. Thus, the antiradical activity of the extract was higher than β-carotene.
The DPPH radical scavenging activity of microbial carotenoid extracts had been reported in the study of Arulselvi et al. (2014) in which carotenoids extracted from bacterial strain YCD3b had quenched 78% radical. In addition, research by Mukherjee et al. (2017) showed that carotenoid extracts could scavenge DPPH radicals follow a dose-dependent manner. Particularly, extracts from bacterial strains YY, TP, and RP (at the total carotenoid concentration of 4 μg/mL) reduced 47.9, 60.3, and 60.0% radical, respectively. TP and RP strains contain deoxyflexixanthin while the YY strain produced zeaxanthin.
Reducing power assay
The presence of antioxidants resulted in the reduction of the Fe3+/ ferricyanide complex to the ferrous form by donating an electron. Fe2+ was then monitored by measuring the formation of Perl’s Prussian blue. The result in Figure 4 shows the ability of β-carotene to reduce Fe3+ ions at different concentrations.
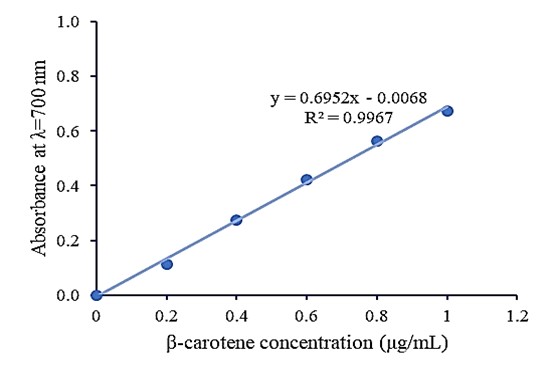
Figure4. Reducing power of β-carotene
β-carotene reduced Fe3+ ions in a dose-dependent manner. The average absorbance value at 700 nm recorded at concentrations from 0.2; 0.4; 0.6; 0.8; 1.0 μg/mL was 0.111; 0.275; 0.422; 0.565; 0.672, respectively. The reducing power of carotenoid extract from Bacillus infantis ND4-4 is 0.29 μg/mL described in the equivalent concentration of β-carotene (μg/mL) through the linear equation of absorbance against concentration.
The research from Vu Thanh Thao et al. (2011) investigated extracts from several Bacillus isolates in which, canthaxanthin was detected in strain DD1.1 extract (B. marisflavi), astaxanthin in AT14 (B. infantis), AT22 (B. licheniformis) and other carotenoids in xanthophyll group accounted for a high percentage. On the other hand, Müller et al. (2011) reported the reducing power of some common carotenoids, in which xanthophyll could reduce Fe3+ better than carotene such as α-carotene, and β-carotene. Xanthophyll had functional groups at position 3(3') such as canthaxanthin, astaxanthin can reduce (approximately 0.3 mol α-TE/mol) and was lower than the xanthophyll functional groups at position 4(4') such as β-cryptoxanthin, lutein, zeaxanthin (about 2.0 mol α-TE/mol). Tan et al. (2014) also got similar results. Lutein had the highest reducing activity, with an absorbance at 700 nm wavelength is about 1.4 while the remaining carotenoids only reach a value <1.2.
β-carotene identification
From the silica gel glass column, the fraction with the yellow-orange color and maximum absorbance from 454 – 455 nm was colleted for β-carotene examination (Britton et al., 2004; Butnariu., 2016). The sample was ionized by ESI giving a principle peak at m/z 537.39, belongs to [M+H]+ and the other ion with m/z 444.33 is [M-92] (Figure 5). The mass spectrum showed that the yellow-orange sample could be β-carotene with the molecular formula C40H56.
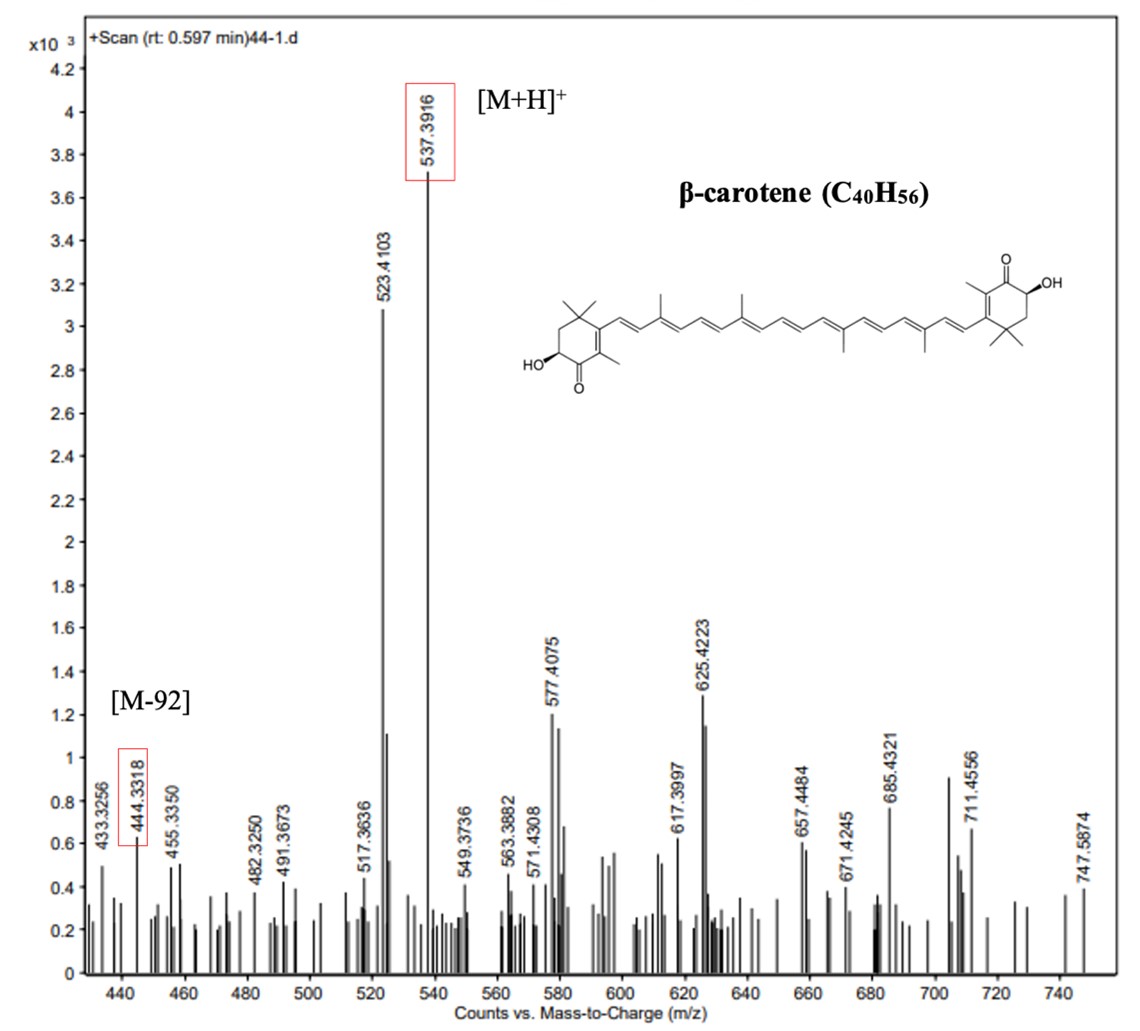
Figure 5. Mass spectrum of F1 fraction from the carotenoid extract of Bacillus infantis ND4-4
Ranga et al. (2009) identified β-carotene in the carotenoid extract from Green Alga Haematococcus pluvialis by HPLC and LC-MS with approximate molecular mass at 536.43 and fraction mass at 537.51 ([M+H]+). Oxley et al. (2014) developing an LC/MS/MS method to study plasma responses of vitamin A and β-carotene in humans showed that APCI of β-carotene resulted in protonation of the molecule [M+H]+ with an abundant Q3 product ion at m/z 177 irrespective of isotopic composition (m/z 537→177 [12C] and m/z 547→177 [13C]).
The benefits of β-carotene to human health have been proven, such as activating certain types of immune cells in the body and reducing DNA damage. According to Fiedor & Burda (2014), supplementation with 20 mg of β-carotene per day for six years significantly reduces the risk of lung and other cancers in male smokers. The addition of β-carotene also protects the skin against sunlight and inhibits the formation of skin tumors. According to Kardinaal et al. (1993) and Gaziano (1994), β-carotene also reduces the risk of cardiovascular disease.
Conclusion
In this study, Bacillus infantis ND4-4 was isolated from the soil of Nam Du islet in Kien Giang Province. Carotenoid extract from Bacillus infantis ND4-4 showed positive activity in scavenging DPPH free radicals and in the reduction of Fe3+ ions. It is worth noting that Bacillus infantis ND4-4 (IC50 = 1.92 μg/mL) had better antiradical activity than β-carotene, but reducing power with the equivalent value is only 0.29 μg/mL. Carotenoids extract show three fractions after purification by glass chromatography column and F1 was identified to be β-carotene by HPLC-MS.
Acknowledgment
We thank the Institute of Food and Biotechnology of Can Tho University for the provided facilities to complete this study.
References
Bergey, D. H. Bergey's manual of determinative bacteriology. Lippincott Williams & Wilkins. 1994.
Holt, J. G. The shorter Bergey's manual of determinative bacteriology. Williams & Wilkins Co., 428 E. Preston Street, Baltimore, Md. 21202, USA. 1977.
Thao, V. T.; Tam, T. H.; Đao, T. T., & Dong, T. C. Levels of carotenoids produced during vegetative phase of carotenogenic Bacillus species. Y Hoc TP. Ho Chí Minh. 2011, 15(1): 211-217 (Vietnamese with English abstract).
The above documents were reviewed and edited by editorial board of NATPRO8 Conference.
Copyright information
© The Asian Society of Natural Products