Introduction
Kratom (Mitragyna speciosa) is an indigenous herbal plant commonly found in Southeast Asia. Its leaves were traditionally known to relief several ailments, such as muscle pain, diarrhea, stomachache, fever, cough, diabetes, hypertension, and opium addiction. Also, the leaves were used for mood and stamina enhancement for outdoor workers.1,2 It was reported that lower dose of consumption provides stimulant effects; however, higher dose leads to opioid-like and psychosis symptoms.3,4 The major compounds that contribute to pharmacological effects of M. speciosa leaves are alkaloids, of which more than 50 compounds were previously characterized.5,6
The most abundant active compound is Mitragynine, which is an indole-alkaloid accounting for about two-thirds of the total alkaloid content.1 Mitragynine and related compounds have been reported to contribute to analgesic effects and opioid agonist activity.7 Various extraction methods with different extraction yields have been reported. Most of the published reports employed organic solvent extraction, including hexane, chloroform, methanol, and ethanol, using Soxhlet, maceration, ultrasonication, and sequential extraction methods. The extraction yield of mitragynine ranged from 0.4 mg/g to 75 mg/g.8,9 A limited number of studies reported the use of aqueous extraction, and the yield was shown to be lower than using solvent extraction. Parthasarathy et al. (2013) reported that methanolic extracts provided a 6-times higher yield of mitragynine than aqueous extract.10
Since mitragynine has a broad range of pharmaceutical properties, this compound is of interest for food-related product development. Therefore, it is important to avoid petrochemical solvents during the extraction process and production. This study focused on the use of water and ethanol, which is considered bio-solvent, for mitragynine extraction. The method for aqueous and ethanolic extraction was optimized to increase mitragynine yield. Furthermore, antioxidant activities of the extracts with a higher yield of mitragynine were evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays. The information from this work would be beneficial for the future development of functional food products with pharmaceutical properties.
Materials and Methods
Plant extract preparation
M. speciosa (red veined leaves) were harvested from the plantation in Pathum Thani province. The leaves were washed with distilled water and air-dried at room temperature. The petioles were removed from the leaves prior to grinding. The leave powder was kept at 4oC.
Extraction of mitragynine
The leaf powder was subjected to 13 different extraction methods as shown in Table 1. For Methods A, B, C, D, E, F, G, H, and I, 10 g of the leaves powder was immersed in 10 mL H2O and incubated in a water bath for 30, 45, and 60 min at 60, 80 and 95 C (Table 1). For Methods G, H, and I, the leaves powder (10 g) was immersed in 10 mL absolute ethanol for 60 min, 1 day, and 3 days at room temperature (Table 1). For Method M, the leaf powder was soaked in 40% ethanol and incubated for 1 day at room temperature prior to incubation at 100C for 30 min. After that, the extracts were filtrated using Whatman® No.1 filter paper. The filtrates were dried using a centrifugal evaporator (SC100, Savant, USA). Percent yield was determined using Equation 1. The extracts were further subjected to the determination of mitragynine concentration using HPLC.
% yield = (weight of the extract/ weight of leaf powder) / 100 Equation (1)
Table 1. Extraction conditions of the Method A-M.
|
Extraction Method |
Conditions |
||
|
Solvent |
Temperature (C) |
Time |
|
|
A |
H2O |
60 |
30 min |
|
B |
H2O |
60 |
45 min |
|
C |
H2O |
60 |
60 min |
|
D |
H2O |
80 |
30 min |
|
E |
H2O |
80 |
45 min |
|
F |
H2O |
80 |
60 min |
|
G |
H2O |
95 |
30 min |
|
H |
H2O |
95 |
45 min |
|
I |
H2O |
95 |
60 min |
|
J |
Absolute ethanol |
Room temperature |
60 min |
|
K |
Absolute ethanol |
Room temperature |
1 day |
|
L |
Absolute ethanol |
Room temperature |
3 days |
|
M |
40% ethanol |
Room temperature, 100 |
1 day, 30 min |
Determination of mitragynine concentration
The concentration of mitragynine from different extraction methods was evaluated by a reverse-phase HPLC following the method from Mudge and Brown (2017) with modifications.11 Briefly, the extract powder was dissolved in acetonitrile, and 20 μL of the solution was subjected to an Agilent 1260 Infinity II HPLC system connected with a diode array detector (Agilent Technology, Wood Dale, IL, USA). A Phenomenex Luna C18 (150 mm x 4.6 mm, 5-μm) column was used for reverse-phase separation. The flow rate was set to 1 mL/min. Isocratic elution was performed using 50% solvent A (0.05% Formic acid in H2O, pH = 5.0) and 50% solvent B (acetonitrile) for 12 min. Mitragynine elution was monitored at 226 nm (bandwidth = 4 nm). The standard mitragynine (CAS Number 4098-40-2) was purchased from Cayman Chemical (Ann Arbor, MI, USA), and the standard concentration varied from 2.5 to 40 μg/mL.
DPPH radical scavenging assay
DPPH radical scavenging assay was modified from Molyneux (2006).12 Kratom leaf extracts (50 μL) with concentrations of 50, 100, 150, 200, 250, 300, 350, and 400 μg/mL were mixed with 100 μL of 0.2 mM DPPH in methanol in 96 well-plate. The mixtures were incubated at room temperature for 30 min prior to absorbance measurement at 517 nm using a VersaMax microplate spectrophotometer. The ability of the leaf extracts to scavenge DPPH radical was determined by Equation (2). Ascorbic acid at the concentrations of 2, 4, 8, 12, 14, 16, and 20 μg/mL was used as the positive control.
% inhibition = [(Acontrol – Ablank) – (Asample – Ablank sample)] / (Acontrol – Ablank) Equation (2)
ABTS radical scavenging assay
The ABTS assay was modified from Shalaby and Shanab, 2013.13 Prior to the assay, ABTS solution was prepared at the concentration of 7 mM in H2O and oxidized by 2.45 mM K2S2O8 for 16 h to form ABTS•+ solution. The leaf extracts at the concentrations of 100, 300, 500, 700, and 900 μg/mL were mixed with 1 ml ABTS•+ solution. The reaction was performed for 6 min at room temperature, and absorbance at 734 nm was recorded. ABTS radical scavenging ability was determined by Equation (3). Ascorbic acid at concentrations of 120, 240, 360, 480, and 600 μg/mL was used as the positive control.
% inhibition = [(Acontrol – Ablank) – (Asample – Ablank sample)] / (Acontrol – Ablank) Equation (3)
Statistical analysis
All experiments were performed in triplicate. The results were shown as mean ± standard deviation. Statistical analyses were performed by Minitab17 using one-way ANOVA with Tukey's Post-test (p < 0.05).
Results& Discussion
The efficiency of 13 different water- and ethanol-based extraction procedures were evaluated. The % yield of the extracts was in the range of 3.02 ± 0.21% and 4.44 ± 0.24% (Figure 1). Considering aqueous extraction, the higher temperature tends to provide a higher extraction yield (Method A-I). Using absolute ethanol at room temperature, maceration of M. speciosa for 1 day and 3 days (Method K and L) provided a higher % yield than incubation for 60 min (Method J). Also, using absolute ethanol (Method L) provided a higher extraction yield than 40% ethanol (Method M), using the same period of incubation. However, the overall trend showed that % yields from both aqueous and ethanolic extractions were not statistically different.
The concentration of mitragynine in the extracts was determined by reversed phase HPLC. An example of a chromatogram from mitragynine was shown in Figure 2(A), presenting the retention time at 3.282 min. The calibration curve of mitragynine at the concentration of 2.5 to 40 μg/mL was shown in Figure 2(B). The concentration of mitragynine extracted from 13 extraction methods was revealed in Figure 3. Absolute ethanol-based extraction methods (Method J, K, and L) were shown to be more effective than 40% ethanol and water-based procedures. In agreement with other published reports that the presence of a lower polarity solvent could enhance mitragynine extraction as compared to water due to the chemical structure and polarity of the compound.10, 14 Method K, which employed maceration of the leaves in absolute ethanol at room temperature for 3 days, was shown to be the most effective method for mitragynine extraction. It provided 39.53 ± 0.34 mg mitragynine/ g extract, which this concentration was in between the values reported in the literature ranging from 0.4 mg/g to 75 mg/g.8, 9 And it could be accounted for 1.59 ± 0.04 mg mitragynine/ g fresh leaf powder. The result from hot water extraction was also in accordance with the previous report. Mitragynine concentration from this work ranged from 0.50 ± 0.10 to 1.41±0.15 mg/ g (Figure 3); while Parthasarathy et al. (2013) revealed 0.80 ± 0.11 mg/g from aqueous extraction.10
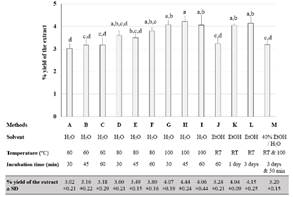
Figure 1 % Yield of M. speciosa leaf extracts from 13 different extraction methods
The ethanolic extract from Method K was chosen for the determination of antioxidant activities using DPPH and ABTS radical scavenging assays. M. speciosa leaf extracts showed the ability to quench DPPH and ABTS radicals in a concentration-dependent manner (Figure 4(A) and Figure 4(B)). The half maximal effective concentration (EC50) values against DPPH and ABTS radicals were 186.59 ± 4.42 and 636.70 ± 9.52 μg/ mL, respectively. The difference in these EC50 values resulted from different mechanisms of action in these two assays. It was suggested that quenching the DPPH radical could be involved with hydrogen atom transfer, whereas ABTS radical scavenging could be via direct electron transfer.15 The EC50 value against DPPH radical of ethanolic extract from our work was in between those from aqueous and methanolic extracts reported by Parthasarathy et al. (2009), which were shown to be 213.4 and 104.81 μg/mL, respectively.16 The radical scavenging abilities of the leaf extracts from both DPPH and ABTS assays were lower than that of ascorbic acid, which was used as a positive control. The EC50 values of ascorbic acid against DPPH and ABTS radicals were revealed to be 19.99 ± 0.40 and 320.30 ± 2.51 μg/ mL, respectively.
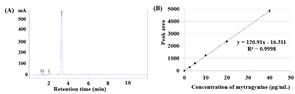
Figure 2. (A) Chromatogram showing mitragynine retention time at 3.282 min. (B)
Standard curve of mitragynine from triplicate experiments.
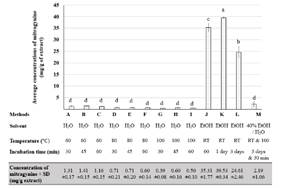
Figure 3. Comparison of mitragynine concentrations (mean ± SD) obtained from different extraction methods. The bar charts with different letters (a, b, c, d) indicate significant differences between the samples (ANOVA and Tukey test, p<0.05).
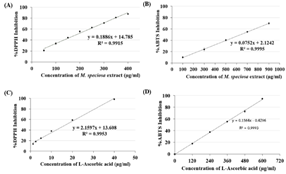
Figure 4. Antioxidant activities of M. speciosa extract at different concentrations were determined by (A) DPPH radical scavenging assay and (B) ABTS radical scavenging assay. And antioxidant activities of ascorbic acid solutions were evaluated by (C) DPPH radical scavenging assay and (D) ABTS radical scavenging assay.
Conclusion
This study revealed that mitragynine could be extracted from M. speciosa leaves using water and ethanol. The most effective condition was using maceration in absolute ethanol at room temperature for 1 day. And this ethanolic extract possessed antioxidant activities against DPPH and ABTS assays. Although the yield of mitragynine from aqueous and ethanolic extraction was shown to be lower than other organic solvent extractions, our method provided the advantage of using a green and non-toxic solvent which could be advantageous for food-based product development.
Acknowledgement
M. speciosa was supported by K.G.M. Thai Herb Co., Ltd. and Good Green Herb Co., Ltd. The authors would like to acknowledge the Science Innovation Facility, Faculty of Science, Burapha University, and the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation for the instrument and financial support.
References
4. Prat, S. S.; Rizvi, S. A.; Chaimowitz, G. A. Kratom-induced psychosis: Case report and literature investigation. Int J Risk Recov, 2020, 3, 29–34. DOI:10.15173/ijrr.v3i1.4134.
12. Molyneux, P. The use of stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004, 26, 211–219.
13. Shalaby, E. A.; Shanab, S. M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J Geomarine Sci. 2013, 556–564.
14. Ditthapornset, S.; Lerkkasemsan, N.; Areerat, S. Solvent selection for mitragynine extraction by hansen solubility parameter. Ladkrabang Eng J. 2023, 40, 72–84.
The above documents were reviewed and edited by editorial board of NATPRO8 Conference.
Copyright information
© The Asian Society of Natural Products