Introduction
Kratom or Mitragyna speciosa Korth (Figure 1) is a member of Rubiaceae family. It was recently taken off of Thailand narcotic plant list in 2021. Before being legally noted as narcotic plant it was recognized as a household plant by Thai people and used for physical endurance and muscle pain relief [1] due to its muscle relaxant property [2]. The director of Thai Traditional Medicine Department, Ministry of Public Health, Thailand recently declared that Kratom had the following properties; pain relief, antidiarrheal, blood sugar control and CNS stimulation [3].

Figure 1. The leaves and plant of Kratom (Mitragyna speciosa Korth).
Mitragynine (Figure 2), an indole major alkaloid, was isolated and identified since 1921 [4], it has molecular structure C23H30O4N2 with molecular weight of 398.503 g/mole. It is soluble in alcohol, chloroform and acetic acid [5].
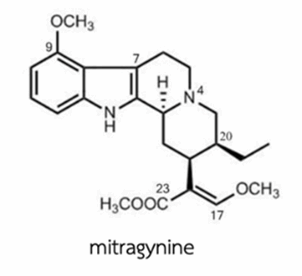
Figure 2. The structure of mitragynine.
The presence of other groups of compounds were also reported, these were flavonoids, triterpenes and phenolic acids [6]. Many pharmacological studies demonstrated that mitragynine had CNS stimulant effects [7], antinociceptive [8, 9], a similar effect on isolated ileum of guinea pig as morphine [9], anti-inflammatory [8], immunostimulant and wound healing [10]. The mechanism of anti-inflammatory action was reported to involve cyclooxygenase 2 and prostaglandin E2 [11]. The ethanolic extract exerted analgesic effect in mice but the aqueous extract did not have the activity which was due to higher amount of mitragynine and flavonoids in the ethanolic extract, therefore the ethanolic extract has been recommended as a potentially effective analgesic [9]. Mitragynine could stimulate the nervous system similar to cocaine [12], administration of mitragynine acetate at a dose of 50 mg in 5 men gave the similar results but also caused nausea and vomiting [7, 13]. However, chewing 2-3 fresh Kratom leaves 3-10 times per day by 30 Thai male workers for 5 years could enhance their work efficiency with minor side effects such as dry mouth, constipation, loss of appetite and weight reduction [1]. From these studies the use of kratom extract was suggested to produce better efficacy than using pure mitragynine [6].
In the United States, many consumers are turning to kratom (Mitragyna speciosa) for self-management in pain and opioid addiction. Many forms of kratom products are readily available such as capsules, powders, and loose-leaf. Moreover, live kratom plants can be bought from several online sites. To understand how these products effect clinical outcomes, it is a prerequisite to establish quality control for the identification and quantitation of active alkaloid constituents within available products. An ultra-high performance liquid chromatography-high resolution mass spectrometry method was developed for the analysis of indole alkaloids content in these preparations available in the USA. These commercial products shared a qualitatively similar alkaloid profile, with 12 - 13 detected alkaloids and high levels of the indole alkaloid mitragynine (13.9 ± 1.1 - 270 ± 24 mg/g) [14].
The different strains of Kratom were noted from leaf vein colours by North American consumers, for more energetic individuals the white and green veins were recommended, while red vein Kratom offered a more relaxed mood and acts as a sedative when taken in large dose. The recommended starting dose is 2 g and with potential increase to 5-15 g. Doses and effects depend on each individual. The expected effects are pain relief, euphoria, relaxation, drowsiness and relief from opioid withdrawal symptoms [15].
An interesting survey conducted in 2017 found that the most beneficial effects were observed in doses of 1–3 and 3–5 g if taken 2–3 times per day; in contrast, most adverse effects required higher doses of >8 g and higher frequency of dosing between 4–5 times per day of daily use [16]. A more recent survey in 2022 reported a consumption of 6.85 g powder was effective while 8.68 g was “a bit too much”. This was followed by 7.25 capsules (“too much”) versus 5.88 capsules (effective); 3.93 spoonfuls (“too much”) versus 2.87 (effective); and 3.44 cups of tea (“too much”) versus 2.25 (effective) [17].
At present, Kratom are still being used in Thailand as single herb or in combination with other botanicals. Over 100 products are available and have been recorded [18]. The uses of Kratom by folk healers in 14 provinces of southern Thailand were as follows; diarrhea (67.4%), diabetes (63.3%), pain relief (32.7%) and cough suppressant (26.5%) [6]. Traditional uses of Kratom were continually used in Kiriwong Temple in Chumporn province, south of Thailand. Among 50 recipes there are 4 recipes that utilize a 40% alcoholic extract of Kratom leaves for topical muscle pain relief [19].
At present it is not clear how much mitragynine is present in these products. It is therefore the objective of this study to develop a validated HPLC method for mitragynine for quality control purpose of Kratom extract.
Materials and Methods
Plant Material
The fresh leaves of Kratom, red vein, were collected from Chumporn province. The herbarium specimen was identified as Mitragyna speciosa Korth by the Thai Traditional Medicine Herbarium, Bangkok and kept under ttm no.0005467.
Chemicals
The solvents and chemicals were all AR grade from Merck, Germany (methanol, glacial acetic acid, n-hexane, 28% ammonia, Silica gel G (9385) for column chromatography, TLC plate silica gel GF254 (105554). Chloroform and ethyl acetate were from Sigma Aldrich, USA, Deionized water was from RCI Labscan, Ireland. Mitragynine Reference Standard was from Chromadex, USA.
The HPLC system consisted of a Thermo, Ultimate 3000 series, Germany, with Photo diode array detector and automatic injector. The system was controlled and data analyses were performed with Thermo Scientific Chromeleon Chromatography Data System (CDS) software. The analysis was done on a Thermo, AcclaimTM 120, C-18 column (5 µm, 4.6 mm x150 mm). All solvents for the mobile phase and sample were filtered through a 0.45 µm nylon filter disc. HPLC analysis for mitragynine was performed by isocratic elution with a flow rate of 0.5 mL/min. The temperature was controlled at 35oC. The detector wavelength was set at 254 nm. The mobile phase was Methanol: Water: Acetic acid (95:4:1, v/v). A volume of 10 µL of each prepared solution and samples were injected into the column. The chromatographic run time was 7 min.
Preparation of sample solution for method validation
Fresh Kratom leaves (10 kg) were washed and dried in a hot air oven at 45 ºC yielded 2,602.86 g dried leaves. Then powdered and sieved through 60 mesh yielded 2,335.25 g Kratom powder. A 200 g of powdered Kratom was macerated in 2000 mL of 95% EtOH for 5 days with occasional stirring. After filtration, the ethanol extract was evaporated in a rotary evaporator at 50 ºC, 60 rpm, followed by a freeze drying process.
The freeze-dried powder was defatted with n-hexane, then the total alkaloid was extracted by acid-base shaking using 10% acetic acid and ammonia (28%). The alkaloid was extracted from the ammoniacal solution (pH 9-10) with chloroform in a separatory funnel. The chloroform layer was evaporated in a rotary evaporator at 50 ºC to provide total alkaloid.
The separation of mitragynine
The separation was done by column chromatography (4 cm x 40 cm) using silica gel 9385 (50 g) as the adsorbent and hexane: ethyl acetate (60:40) as the developing solvent. The total alkaloid was dissolved in 5 mL chloroform and added to the column. The elution was made at the rate of 5 mL/min and collected 10 mL/tube yielded 60 fractions. The fractions 21-32 contained mitragynine as the major component by TLC (silica gel GF254, CHCL3: MeOH 95:5). This fraction was further separated in the second column using silica gel 9385 (25 g) as the adsorbent and CHCL3: MeOH (97:3) as the developing solvent. The fractions 21-32 from the first column was dissolved in chloroform and added to the second column. The elution was made at the rate of 5 mL/min and collected in 9 mL/tube yielded 25 fractions. The fractions 13-21 contained mitragynine as the major component by TLC (silica gel GF254, CHCL3: MeOH 97:3). This fraction yielded picrate with saturated solution of picric acid and was used for HPLC in method validation.
Preparation of Standard Solution
Prepare mitragynine solution at 200 µg/mL in methanol by accurately weighed 1 mg of mitragynine dissolved in 5 mL methanol, then diluted to 100, 50, 25, 12.5, and 6.25 µg/mL. The solutions were filtered through 0.45 µm nylon filter disc before injection.
Method Validation [20]
Specificity: The standard solution of mitragynine at 50 µg/mL was injected to HPLC to observe the shape, separation and retention time of the peak.
Linearity: Each diluted solution of mitragynine at 6.25, 12.5, 25, 50, 100, 200 µg/mL were injected 3 times and the HPLC chromatograms were recorded. The standard curves were prepared by plotting the concentrations (X-axis) against peak areas (Y-axis). The coefficient of determination (r2) must not be less than 0.9950.
Precision: Intra-day Precision. The solution of mitragynine at 3 concentrations; 12.5, 25 and 50 µg/mL were injected 7 times within the same day. The percentage of Relative Standard Deviation (% RSD) were calculated (Equation 1), the value should not be more than 2%.
Inter-day Precision. The solution of mitragynine at 3 concentrations; 12.5, 25 and 50 µg /mL were injected 7 times in 3 consecutive days. The percentage of Relative Standard Deviation (% RSD) were calculated (Equation 1), the value should not be more than 2%.
% RSD = Standard Deviation X 100 (equation 1)
![]()
mean
Standard Deviation = ![]() (equation 2)
(equation 2)
Xi = data
n = replicate
Accuracy: The accuracy of the method was done by addition of standard solution of mitragynine (50 µg/mL) to sample solutions in set 2.
Sample solution set 1: Prepare sample solution at 100 µg/ml in 10 ml volumetric flask, then pipette 2.5 1.5, 0.625 mL into 3 x 5 mL volumetric flasks to give 50, 30 and 12.5 µg/ml respectively.
Sample solution set 2: Prepare sample solutions in 3 x 5 mL volumetric flasks as in set 1, then add standard solution of mitragynine (50 µg/mL) to the volumetric flasks, make up volume to 5 mL.
% recovery = (S2) – (S1) x 100 (equation 3)
(S2)
where S1 = data from set 1
S2 = data from set 2
Limit of Detection (LOD) and Limit of quality (LOQ): The value of LOD and LOQ were calculated from the graph (equation 4, equation 5).
LOD = 3.3 σ/S (equation 4)
LOQ = 10 s/S (equation 5)
where s = the standard deviation of y-intercepts.
S = the slope of the calibration curve
Preparation of freeze-dried 40% ethanol extract for analysis
Ten milligrams of freeze-dried powder of 40% ethanol extract was dissolved in 10 mL methanol to give 1000 µg/mL concentration. From this solution an aliquot of 0.2 mL was pipetted into a 5 mL volumetric flask and volume was adjusted with methanol to give 40 µg/mL analytical concentration. The solution was filtered through 0.45 µm disc prior to HPLC injection (n=3).
Results& Discussion
The developed HPLC method offered shorter time of analysis i.e. 7 min vs 10 min in prior study [21]. In this study mitragynine was detected at 254 nm and appeared at 3.23 min sooner than previous study at 5.97 min (Figure 3). This could be due to the use of C-18 column instead of C-8 column also acetic acid was used and the temperature of column was kept at 35oC.
Preparation of sample solution for method validation
From 200 g of powdered Kratom yielded 48.28 g freeze-dried powder. The powder (12 g) was defatted with n-hexane and left to dry, yielded 9.8622 g defatted powder. Acetic acid (10%) was added to defatted powder and filtered. The acidic filtrate was extracted with n-hexane and discarded. The aqueous acidic layer was then basified to pH 9-10 with ammonia (28%) and the total alkaloid was extracted with chloroform. After solvent evaporation the yield of total alkaloid was 1.29 g or 0.645% of Kratom dried powder.
Mitragynine was separated from the total alkaloid by two consecutive column chromatography procedures and kept in the form of picrate (0.0397 g). Mitragynine was extracted back from this picrate (10 mg) by acid-base shaking yielding 0.0064 g alkaloid which was dissolved in 5 mL methanol giving 1.28 mg /ml. This solution was used in method validation.
Method Validation Parameters
Specificity: The retention time of mitragynine in the standard solution and in the sample was 3.23 minutes (Figure 3 and Figure 4).

Figure 3 The HPLC chromatogram of standard mitragynine.

Figure 4. The HPLC chromatogram of mitragynine in sample solution.
Linearity
The linear relationship between peak area and concentration of standard mitragynine was found to be in the range 6.25 - 200 µg/ml. The correlation coefficient (r2) from equation y = 0.6x-0.9901 was equal to 1 which passed the requirement of ICH guideline (> 0.995) (Figure 5).

Figure 5. The linear regression line of standard mitragynine.
Precision
According to ICH guideline precision means that all measurements of an analyte should be very close together. There should be no more than a ±2% variation in the assay system. A useful criterion is the relative standard deviation (RSD) or coefficient of variation (CV), which is an indication of the imprecision of the system (Equation 1). This study was performed with 3 concentrations of mitragynine standard at 50, 25 and 12.5 µg/ml within the same day (intra-day precision) showing %RSD to be 0.334, 0.178 and 0.355 respectively. The analysis of the 3 standard solutions in different days (inter-day precision) showed the %RSD to be 0.200 0.234 and 0.214 respectively. The results demonstrated that this method provides precision for the analysis within the same day or in different days.
Table 1. The analytical results from intra-day precision and inter-day precision study.
|
Mitragynine (µg/mL) |
Intra-day Precision |
Inter-day Precision |
||||
|
Mean |
SD |
%RSD |
Mean |
SD |
%RSD |
|
|
50 |
50.534 |
0.169 |
0.334 |
50.081 |
0.100 |
0.200 |
|
25 |
24.950 |
0.044 |
0.178 |
24.723 |
0.058 |
0.234 |
|
12.5 |
11.694 |
0.041 |
0.355 |
11.657 |
0.025 |
0.214 |
Accuracy
A method is said to be accurate
if it gives the correct amount for the analyte. However, the results of several
replicate tests may not give the same answer, so the mean or average value is
taken as the estimate of the accurate answer. Accuracy is usually determined by
measuring a known amount of standard material. For assay methods, spiked
samples are prepared in triplicate at three levels, the per cent recovery
should then be calculated. In the present study, the accuracy of the method was
evaluated by adding 25 µg/ml of mitragynine standard to a known amount of
sample, at 12.5, 30 and 50 µg/ml. The samples were analyzed, and mean recovery
calculated. The data presented in Table 2 show that the recovery of mitragynine
in spiked samples met the evaluation criterion for accuracy (80–120%).
Table 2. The percentage of mitragynine recovery in samples with and without mitragynine
standard addition (25 µg/ml).
|
Sample concentration (µg/ml) |
Sample with standard addition (µg/ml) |
Sample without standard addition (µg/ml) |
(%recovery) |
||
|
50 |
64.777 |
41.651 |
92.507 |
||
|
64.782 |
41.012 |
95.078 |
|||
|
64.880 |
41.041 |
95.356 |
|||
|
30 |
46.853 |
22.064 |
99.157 |
||
|
47.053 |
21.976 |
100.307 |
|||
|
47.664 |
22.004 |
102.640 |
|||
|
12.5 |
37.158 |
13.316 |
95.370 |
||
|
37.261 |
13.340 |
95.685 |
|||
|
37.284 |
13.338 |
95.785 |
|||
|
Average |
|
|
96.876 |
||
The limit of detection (LOD) and the limit of quantitation (LOQ)
The limit of detection (LOD) is defined as the lowest concentration of an analyte in a sample that can be detected. It is expressed as a concentration at a specified signal:noise ratio, 3:1. The limit of quantitation (LOQ) is defined as the lowest concentration of an analyte in a sample that can be determined with acceptable precision and accuracy under the stated operational conditions of the method. The ICH has recommended a signal:noise ratio 10:1. LOD and LOQ may also be calculated based on the standard deviation of the response (SD) and the slope of the calibration curve according to equation 4 and equation 5. The results showed that the values of LOD and LOQ for mitragynine determination were 0.266 and 0.807 µg/mL respectively (Table 3).
Table 3. The calculation data for LOD and LOQ of Mitragynine standard.
|
No. of |
Linear equation |
slope |
Y-intercept |
|
1 |
y = 0.5993X - 0.9857 |
0.5993 |
0.9857 |
|
2 |
y = 0.5994x - 0.9441 |
0.5994 |
0.9441 |
|
3 |
y = 0.6013x - 1.0406 |
0.6013 |
1.0406 |
|
Mean |
|
0.6000 |
0.990 |
|
SD |
|
|
0.048 |
|
LOD 0.266 µg/mL |
|||
|
LOQ 0.807 µg/mL |
|||
The content of mitragynine in freeze-dried 40% ethanol extract
The analytical sample was injected three times and the content of mitragynine was calculated based on the developed method. It was found that the freeze-dried 40% ethanol extract contained 24.37% mitragynine equivalent to 33.45 mg/g dry weight of Kratom leaves (Table 4). This finding is much higher than previously reported from HPLC chromatogram, 8.76 mg/g [8].
Table 4. The content of mitragynine in freeze-dried extract of 40% ethanol based on linear regression and its content in dried Kratom leaves.
|
Injection |
Area (Y) |
µg/mL (X) |
mitragynine In freeze dried extract (%w/w) |
Mitragynine In Kratom leaves (mg/g dry weight) |
|
1 |
4.881 |
9.785 |
24.46333 |
33.573 |
|
2 |
4.877 |
9.778 |
24.44583 |
33.549 |
|
3 |
4.819 |
9.683 |
24.20625 |
33.220 |
|
mean |
4.859 |
9.749 |
24.37181 |
33.447 |
|
SD |
|
|
|
0.197 |
|
mean±SD |
|
|
|
33.447±0.197 |
Conclusion
The fact that Thai workers chewed 2-3 fresh Kratom leaves 3-10 times per day for 5 years which could lead to the enhancement of their work efficiency with minor side effects such as dry mouth, constipation, loss of appetite and weight reduction [1] provided evidence of efficiency and safety. This also indicates that Kratom leaves are well tolerated. From our data a Kratom leaf yielded about 1 g of dry weight which would provide 33.45 mg of mitragynine, however, there are other groups of compounds which may contribute to the overall effects of Kratom leaf and its safety. Moreover, the red vein strain Kratom was use in this study which was considered to have a stronger effect than other strains. Whether other strains contain more or less mitragynine content should be investigated in future studies. In addition, the strength of ethanol used for extraction should be delineated. Overall, our findings support the traditional use of 40% ethanol in Kratom extraction.
References
1. Suwanlert, S. A study of kratom eaters in Thailand. Bull Narcotics. 1975, 27, 21-27.
3. PBS News (2021, August 24th). Fatigue-Muscle pain from WFH can be relieved with Kratom leaves (in Thai).
5. Joshi, B.; Raymond-Hamet.; Taylor, WI. Structure of mitragynine (19-methoxycorynan theidine). Chem Ind. 1963, 14, 573.
6. Wungsintaweekul, J. Kratom Plant. Center for Continuing Pharmacy Education. 2017 (in Thai).
7. Grewal, K.S. The effect of mitragynine on man. Br J Med Psychol.1932, 12, 41-58.
15. Kratom dosage guide Kratom dosage guide- everything you should know.
19. Akkachayo, K. The Charm of Kratom and Thai Formula. Thai Folklore herb Learning Center- Mor Porn, Chumporn Province, Thailand. 2022 (in Thai).
20 ICH. Harmonised tripartite guideline. Validation of analytical procedures: Text and methodology Q2(R1). Geneva: International Conference on Harmonisation; 2005.
The above documents were reviewed and edited by editorial board of NATPRO8 Conference.
Copyright information
© The Asian Society of Natural Products