Introduction
Inflammation is a crucial biological response that helps protect the body from infections and injuries and maintains homeostasis. However, chronic inflammation can contribute to the development of various diseases, including rheumatoid arthritis, chronic hepatitis, pulmonary fibrosis, and cancer [1]. The initiation of inflammation involves innate immune cells, particularly macrophages, which release pro-inflammatory mediators such as nitric oxide (NO) and prostaglandin E2 (PGE2), as well as inflammatory cytokines like tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6). Macrophages and dendritic cells play a crucial role in detecting danger signals and triggering an inflammatory response. These cells release pro-inflammatory mediators to promote the migration of leukocytes, eliminate the cause of infection, and contribute to tissue repair [2, 3]. One of the important molecules released by activated macrophages and immune cells is nitric oxide (NO), which has dual functions in cells: homeostasis and cytotoxicity. Under normal physiological conditions, NO is produced in small amounts and serves regulatory functions such as vasodilation, platelet and neutrophil adhesion control, and involvement in neurotransmission. However, in abnormal situations, overproduction of NO can occur, leading to its role as a pro-inflammatory mediator and contributes to the pathogenesis of inflammatory disorders. The excessive production of NO can cause vasodilation and tissue damage during the inflammatory process [4, 5]. Therefore, the development of NO inhibitors represents a significant therapeutic advancement in the management of inflammatory diseases. NSAIDs are effective in reducing inflammation and also possess antipyretic (fever-reducing) and analgesic (pain-relieving) properties [6, 7]. However, their adverse effects, such as gastrointestinal, renal, and cardiovascular toxicity, have restricted their usage. To overcome these limitations, researchers are exploring natural compounds and herbs as alternative options.
Bhup-po is prescribed in clinics for the following symptoms; general wounds, abscess, sore throat, tonsilitis, chronic cough, chronic wounds, diabetic wounds, pressure sores, traumatic injury and long COVID. The main herbs are Ya-Prag or Burmuda grass, Cynodon dactylon (L.) Pers (Poaceae) and Ya-teen-Nok or Fingergrass, Digitaria ciliaris (Retz.) Koel (Poaceae). Mareng formula is used to treat cysts, benign and malignant tumours of the internal organs (lung, liver, spleen, kidney, heart) and breast cancer. It is usually taken with Bhup-po. The main herbs are whole plants of Tong-pan-chang or White crane flower, Rhinacanthus nasutus (L.) Kurz. (Acanthaceae), Krob-jakra-wan or Indian Mallow, Abutilon hirtum and Krob-fun-si or Country mallow, Abutilon indicum (Malvaceae) and Pak-kra-sang or Pepperomia, Peperomia pellucida (L.) Humb., Bonpl & Kunth (Piperaceae). The gastric formula is used in the treatment of peptic and duodenal ulcers and GERD. The main herb is Kamin-oy, or White turmeric, Curcuma zedoaria (Christm.) Roscoe (Zingiberaceae). Sahasa-rangsi formula is used for the tendon and muscle inflammation in rheumatoid arthritis, gout, osteoarthritis, herniated disc, piriformis syndrome, and stiff neck and is usually taken with Bhup-po. The main herbs are Kamin-Kruea or Yellow Fruit Moonseed, Arcangelisia flava (L.) Merr. (Menispermaceae) and Plai, Zingiber montanum (Koenig) Link ex Dietr.
Therefore, the present study focuses, for the first time, on the evaluation of the anti-inflammatory potential of four herbal formulae: Bhup-po, Mareng, Gastric, and Sahasa-rangsi through the inhibition of NO production in LPS-activated RAW264.7 macrophages.
Materials and Methods
Preparation of plant extracts
The powdered of each recipe Bhup-po, Mareng, Gastric and Sahasa-rangsi was extracted for 5 days in 100 mL 95% ethanol. The separated extracts were then filtered through Whatman No. 1 filter paper and the ethanol filtrate evaporated to dryness using a rotary evaporator at room temperature (30 °C). The thick extracted mass was then dried at room temperature, and the dried extract stored in an air-tight container at 4 °C until further use. Percentage yields of the crude extracts were calculated asweight of crude mushroom extract (g)/weight of dry mushroom (g)x100%] [8].
Cell culture
RAW 264.7 macrophages were obtained from the American Type Culture Collection (ATCC). RAW 264.7 macrophages were cultured in 37°C, 5% CO2 incubator with Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich Co.) containing 10% fetal bovine serum (FBS; Gibco), and 1% Penicillin-Streptomycin solution (Sigma-Aldrich Co.), and were split twice a week.
Measurement of Nitric Oxide Production
Nitrate and nitrite concentrations were assayed by Griess reagent using a nitric oxide assay kit as described previously [9]. RAW 264.7 cells (4 × 105 cells/well) were plated in 96-well culture plates overnight. Under the serum-free condition, cells were pretreated with the Bhup-po, Mareng, Gastric and Sahasa-rangsi extract (12.50-200 µg/mL), for 1 h and subsequently treated with LPS (100 ng/mL) for 24 h. After 24 h, the supernatants (100 µL) were transferred into 96-well plates and mixed with 100 μL Griess reagent (20 μL of 1% sulfanilamide in 5% phosphoric acid and 20 μL of 0.1% naphthyl-ethylenediamine dihydrochloride). After 10 min, the absorbance was determined at 550 nm. The concentrations of nitrite were calculated from regression analysis using serial dilutions of sodium nitrite as a standard. Percentage inhibition was calculated based on the ability of extracts to inhibit nitric oxide formation by cells compared with the control (cells in media without extracts containing triggering agents and DMSO), which was considered as 0 % inhibition.
Results & Discussion
Crude yield of extracts
Table 1 presents the percentage yield of ethanolic extracts obtained from Bhup-po, Mareng, Gastric, and Sahasa-rangsi using ethanol as the solvent. The ethanolic extract yields for Bhup-po, Mareng, Gastric, and Sahasa-rangsi were determined to be 8.16%, 6.50%, 8.98%, and 8.14%, respectively.Table 1. Percentage yield of ethanol extracts.
|
Extracts |
% yield |
|
Bhup-po |
8.16 |
|
Mareng |
6.50 |
|
Gastric |
8.98 |
|
Sahasa-rangsi |
8.14 |
Cytotoxicity assessment in RAW 264.7 cells
MTT assay was used to assess the cytotoxicity of each extract in RAW 264.7 macrophages (Figure 1). Sample concentrations with cell viability of ≥90% relative to the control group were considered to be safe, non-toxic concentrations. Cell viability measurement after treatment with 12.50, 25, 50, 100, and 200 μg/ml of each extract showed cell viability of ≥90% up to the concentration of 200 μg/ml, revealing no cytotoxicity. On the basis of these results, the 12.50, 25, 50, 100, and 200 μg/ml samples were selected for further experiments.
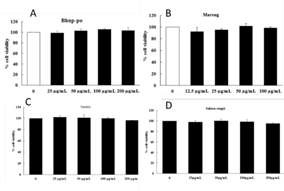
Figure 1 Cytotoxicity of Bhup-po, Mareng, Gastric, and Sahasa-rangsi extracts in RAW 264.7 cells. RAW 264.7 cells were incubated for 24 hr in the presence or absence of each extract at the indicated concentration. Cell viability was evaluated by the MTT assay. Data represent the mean ± SEM of triplicate determinations from three separate experiments (n=3). (A) Bhup-po (B) Mareng (C) Gastric (D)Sahasa-rangsi
Table2. NO inhibitory activity of extracts.
|
Extract |
%
inhibition at various concentrations |
IC50 (µg/mL) |
||||
|
12.50
µg/mL |
25
µg/mL |
50
µg/mL |
100
µg/mL |
200
µg/mL |
||
|
Bhup-po |
|
28.32±2.67 |
63.05±2.56 |
91.53±6.90 |
101.82±0.89 |
40.10±1.46 |
|
Mareng |
30.92±5.12 |
59.22±7.49 |
91.84±0.90 |
96.20±2.68 |
- |
20.77±3.41 |
|
Gastric |
- |
34.05±6.24 |
71.03±0.62 |
92.26±3.47 |
99.47±2.63 |
35.00±5.32 |
|
Sahasa-rangsi |
- |
45.00±6.39 |
75.54±8.38 |
96.11±0.67 |
104.45±6.38 |
33.25±1.80 |
|
Dexamethasone 20 µM , % inhibition= 48.80±5.45 |
||||||
Each value represents the mean ± S.E.M (n=3).
Inhibitory effects on NO production
Lipopolysaccharides are extracellular components of gram-negative bacteria and they act as powerful stimuli for various cells such as monocytes and macrophages. In particular, when macrophages are activated after stimulation by LPS, they produce and release inflammatory mediators such as NO via regulation of pro-inflammatory factors Therefore, in the present study, we examined the effects of Bhup-po, Mareng, Gastric, and Sahasa-rangsi extracts on the production of NO, which is one of the active oxygen species and is known to play a key role in inflammation induction. The study aimed to assess the inhibitory effects of Bhup-po, Mareng, Gastric, and Sahasa-rangsi extracts on nitric oxide (NO) production in LPS-stimulated RAW 264.7 cells. The amount of NO produced was quantified by measuring the NO2 content in the cell culture broth using the Griess reagent. Upon LPS stimulation, untreated cells exhibited an increase in NO production. However, treatment with Bhup-po, Mareng, Gastric, and Sahasa-rangsi extracts effectively inhibited this LPS-induced increase in NO production, and the inhibitory effects were observed to be concentration-dependent (Figure 2).
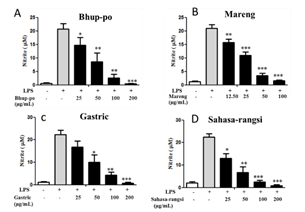
Figure 2. Effect of Bhup-po, Mareng, Gastric, and Sahasa-rangsi extracts on nitrite production in RAW 264.7 cells. After pretreatment with the indicated concentration of each extract for 1 hr, RAW 264.7 cells were treated with 100 ng/ml lipopolysaccharide (LPS) for 24 hr. Nitrite levels in the cell media were measured spectrophotometrically using Griess reagent. Data are provided as mean ± SEM (n=3). *p < 0.05 ***p < 0.01 ***p < 0.001 vs C LPS treatment. (A) Bhup-po (B) Mareng (C) Gastric (D) Sahasa-rangsi.
Four extracts were evaluated for their ability to inhibit NO production. Mareng extract demonstrated robust anti-inflammatory activity, with a significant inhibition of 96.20±2.68% at a concentration of 100 µg/mL, and its half-maximal inhibitory concentration (IC50) was determined to be 20.77±3.41 µg/mL. Sahasa-rangsi extract also exhibited inhibition of NO production, with a notable 104.45±6.38% inhibition at a concentration of 200 µg/mL and an IC50 value of 33.25±1.80 µg/mL. Similarly, Bhup-po and Gastric extracts displayed strong inhibition of NO production, with percentages of inhibition of 101.82±0.89% and 99.47±2.63% respectively at a concentration of 200 µg/mL. The corresponding IC50 values for Bhup-po and Gastric extracts were 40.10±1.46 µg/mL and 35.00±5.32 µg/mL respectively (Table 2).
References
1. Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428-435.
2. Nourshargh, S.; Alon, R. Leukocyte Migration into Inflamed Tissues. Immunity. 2014, 41, 694-707.
3. Coleman, J. W. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001, 1, 1397– 1406.
The above documents were reviewed and edited by editorial board of NATPRO8 Conference.
Copyright information
© The Asian Society of Natural Products